Free rapid antigen tests coming to ECEC in Victoria to minimise learning disruptions
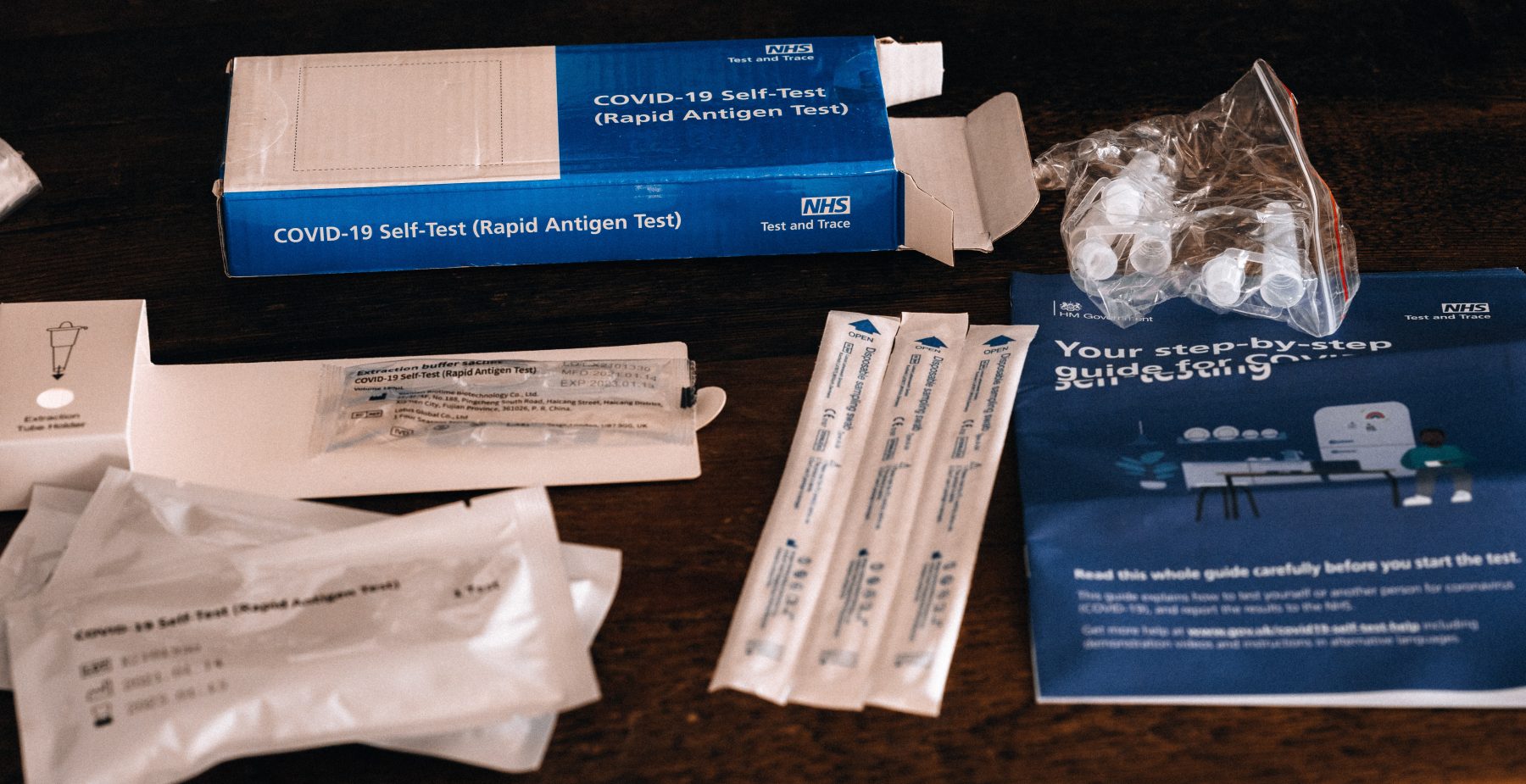
Early childhood education and care (ECEC) services in Victoria will soon have access to free rapid antigen tests to bring them into line with school-based settings in terms of managing outbreaks of the coronavirus, and limiting disruptions to children’s learning.
Effective Monday 15 November, all eligible kindergarten and long day care services are able to opt in to the program to receive at-home rapid antigen test kits for children who have been identified as primary close contacts – reducing both quarantine time and pressure on families, while ensuring early childhood settings are as low-risk as possible.
Test kits will be available to early childhood services subject to outbreaks from today and will be distributed to eligible services by the end of this week, ready to be used from Monday, 22 November.
The rapid antigen tests will be provided for free to kindergarten and long day care services who have a system in place to collect, record and hold the testing information, and parents and carers will opt-in for their children to be part of the scheme.
Once tests have been delivered to services, children can return to their early childhood service after seven days of quarantine if they get a negative standard PCR test on day six at their local testing site, and then return a negative rapid antigen test result each day they attend their early childhood service from days eight to 14.
The quick and easy tests indicate a result for coronavirus within 15-30 minutes, with families required to report the test results to the early childhood service each morning prior to attending – supporting children to return to early learning as quickly as possible.
Home-based rapid antigen testing has had approval from the Therapeutic Goods Administration since Monday, 1 November. The tests indicate whether a person is likely to have coronavirus, but a standard PCR test is still needed to confirm a positive infection.
For more information, please see here.
Popular

Quality
Provider
Policy
Practice
WA approved provider fined $45,000 over bush excursion incident
2025-07-01 07:00:01
by Fiona Alston

Workforce
Policy
Quality
Practice
Provider
Research
ECEC must change now, our children can’t wait for another inquiry
2025-07-02 07:47:14
by Fiona Alston

Quality
Practice
Provider
Workforce
Child left in storeroom at Sydney centre sparks concerns over supervision and trauma support
2025-06-30 09:09:58
by Fiona Alston













